Meet selectION, Inc. at the 44th Annual J.P. Morgan Healthcare Conference in San Francisco, CA, on January 12th-15th, 2026
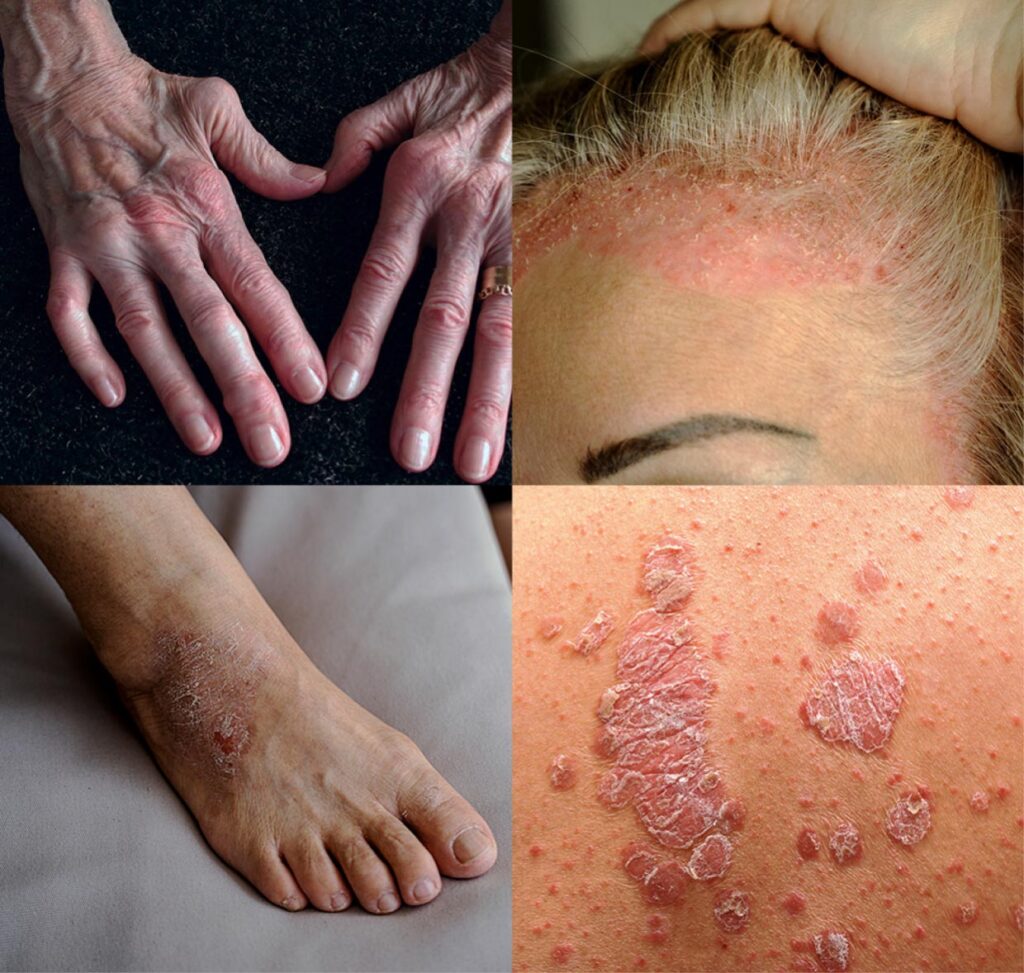
SAN DIEGO, Dec 12, 2025 – Meet selectION’s management team for an update on selectION’s best-in-class Kv1.3 ion channel blocker, si-544, for the disease modifying treatment of T cell-mediated autoimmunity. selectION successfully completed Phase 1b clinical trials in atopic dermatitis and psoriasis patients, demonstrating excellent safety, tolerability and early efficacy signals. Currently selectION is planning subsequent Phase 2 studies.
Read More