SCIENCE
Autoimmunity is an everyday burden
people carry for life.
-
A lifetime of treatment, follow-up visits and tests
-
Serious risks due to immunosuppression under therapy
-
Potential for multiple autoimmunities to present
-
Significant toll on mental health and financial well-being
-
Symptomatic treatments have no significant impact on lifelong disease burden
Immunosuppressive drugs treat inflammation, not the root cause of disease
Autoimmune diseases occur when the immune system mistakenly attacks the body’s own cells, leading to chronic inflammation. Immunosuppressive drugs (including steroids, cytotoxic agents, and biologics) reduce this inflammation by limiting immune cell activity or blocking inflammatory signals. This helps relieve symptoms, decrease tissue damage, and sometimes induce temporary remission. However, these treatments generally do not correct the underlying immune malfunction that triggers autoimmunity. As a result, the disease itself is not cured and regular maintenance therapy is required to control symptoms.
MECHANISM OF ACTION
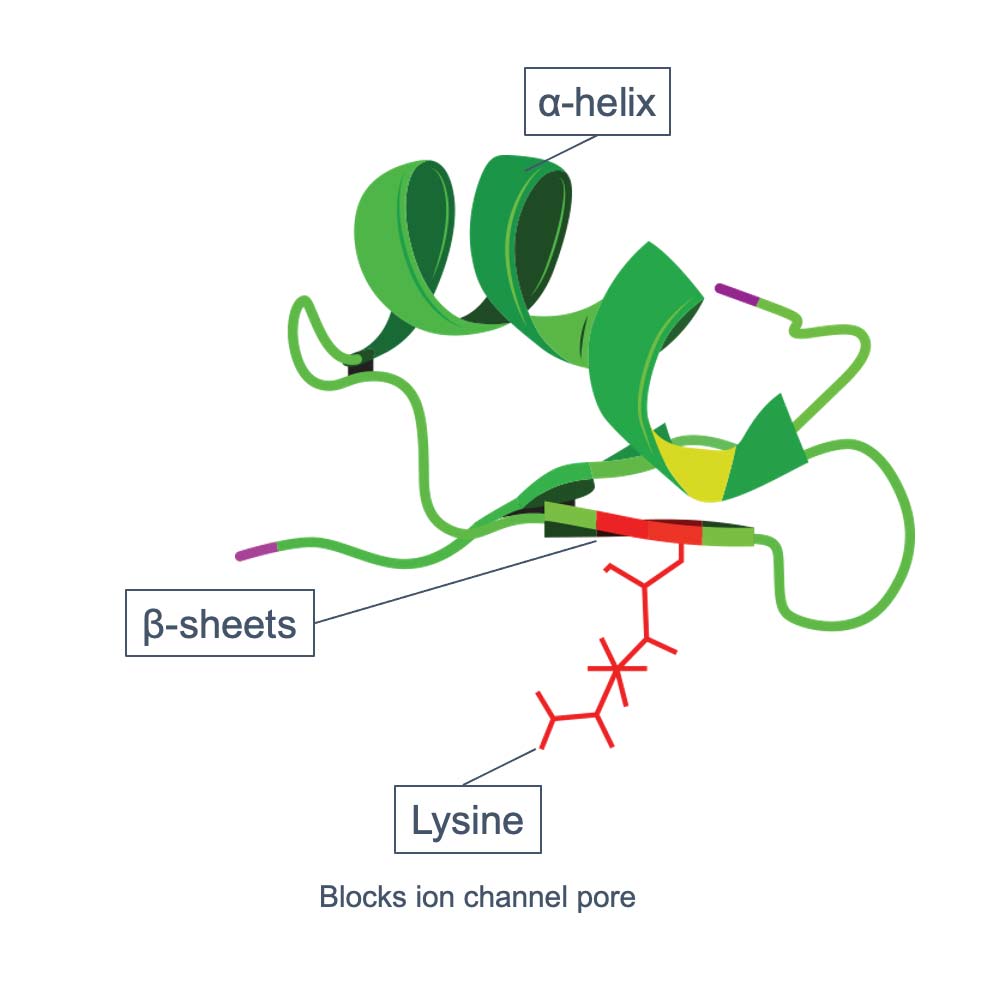
Kv1.3 dependency is fundamental biology in T cell autoimmunity
Autoreactive T cells are Kv1.3 dependent
The Voltage Gated Potassium Channel Kv1.3 plays a Key Role in Numerous Indications
T cells play a cardinal role in the human immune response system. Auto- or alloantigenic recognition or mutations can provoke undesirable immune responses that lead to autoimmune diseases. In such autoimmune diseases a T cell subset called effector memory T cells (TEM) escapes the control of the immune system and destroy the body’s own tissues.
Therapeutic down-regulation of the complete T cell system eases harmful immune response, yet disrupts the protective immune response network. Therefore, it is desirable to focus on TEM cell subsets to maintain the overall protective immune response.
Autoreactive TEM cells become exclusively dependent on the potassium channel Kv1.3 for maintained activation and proliferation. Kv1.3 dependency is unique to these cells and Kv1.3 is therefore regarded as an ideal target for selective and specific deactivation of autoreactive TEM cells.
selectION’s si-544 peptide blocks the Kv1.3 channel with high affinity and specificity. This irreversibly disrupts the signaling pathway necessary for maintaining TEM cell activation and proliferation. Short si-544 exposure terminates the pathogenic activation and proliferation cascade of the autoreactive, disease-associated TEM cell clone, while leaving the remaining immune system untouched.