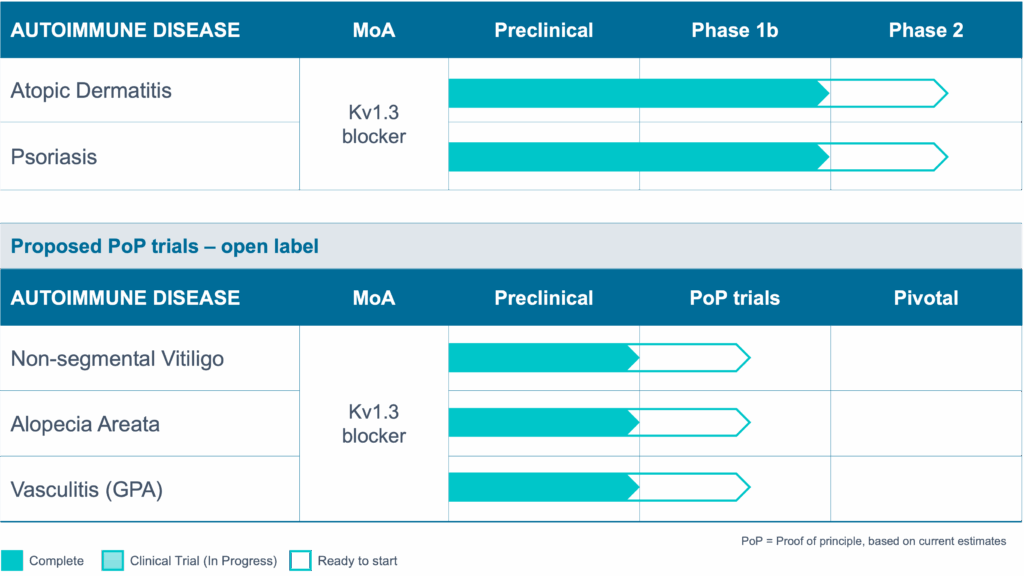
T cell-driven autoimmunity spans 80+ diseases and affects up to 50M people in the US alone. Due to its unique, disease-modifying, mechanism of action si-544 has the potential to positively impact them all.
Common T cell-mediated autoimmune diseases include Atopic Dermatitis, Psoriasis, Rheumatoid Arthritis, Multiple Sclerosis, Inflammatory Bowel Disease, Lupus Nephritis, Vasculitis, and many other indications.